Traveller Rules-as-written (RAW) indicate that starship fuel is liquid hydrogen (LH2). I find this to be a ridiculous concept, as the requirements for cooling or pressuring tons of hydrogen into liquid form are both wasteful and potentially dangerous. Here is how it works in my Traveller universe (IMTU):
Fusion
Power plants IMTU use the Deuterium-Deuterium (D-D) fuel cycle as its base. This produces less power than the Deuterium-Tritium (D-T) cycle -- 3.2-4 MeV versus the D-T's output of 17.5 MeV -- but it has the twin virtues of not requiring expensive/dangerous/time consuming Tritium breeding and needing only a single universal fuel.
Therefore, the Deuterium fusion cycle of a typical Traveller powerplant goes something like this:
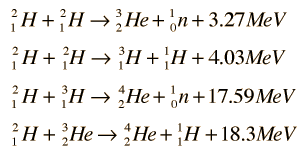 |
| Apologies for the crap resolution. If you click through it will be readable. |
Or, as a single line,
(For the curious, 43.2 megaelectron volts = 6.92140232 × 10-12 joules. I'll be honest and admit that I don't really know what these numbers mean, as I pulled them from various articles on the web, but they look large enough that my non-engineer brain has no problem accepting them as suitable output for a starship. If you're an engineer and you spot something wrong with any of my assumptions, please let me know.)
Fuel Refinement
However, since Deuterium is less than 0.02% of all naturally-occurring hydrogen, it needs to be distilled ("refined," in Traveller parlance) before it can be used. Fortunately, Deuterium is stable and non-radioactive -- unlike Tritium, which is (mildly)radioactive and has a half-life of 10-12 years.
So these are the basic procedures of fuel refinement:
- Take in a liquid or gas with a sizable Hydrogen component (ocean dipping, gas giant skimming, crushing iceball asteroids);
- Crack it into its components, saving useful elements (oxygen) and expelling the waste;
- Separate the Deuterium from regular Hydrogen (a process which can take quite some time);
- Store it in liquid form for later use (see next section).
This tells us some interesting things:
- A fusion reactor that needs to be cold-started cannot use unrefined fuel, because by my definition unrefined fuel is unsorted molecular hydrogen, and power is needed to refine the Hydrogen into Deuterium.
- Contrary to what RAW says, small craft cannot use unrefined fuel unless they have onboard fuel processors.
- This also answers the unasked question "Why would anyone buy refined fuel?" Well, you need it for your small craft and cold-starting reactors.
- This also has the side-effect of requiring class C starports to carry refined fuel, albeit in limited supplies. I don't see this as a problem, because nearly all C ports have fusion power anyway. If you intend to buy in quantities larger than small-craft fuel tanks, you can expect to pay out the nose for it.
Fuel Storage
Even though Deuterium is stable and non-radioactive, it's still hydrogen and therefore explosive. Just the kind of thing you want sitting around a busy starport in large tanks, or surrounding the crew and ammunition in a starship, right?
Therefore IMTU, fuel which is in the tank (as opposed to being in the reactor) is converted to a more stable form. Civilian ships* convert it to ammonia for storage - specifically, triply deuterated ammonia, which is three atoms of Deuterium bonded to one atom of Nitrogen.**
Ammonia is poisonous, yes, but it has the following benefits over liquid Hydrogen:
- It's not explosive.
- It doesn't need compression or refrigeration to be a liquid.
- It's not explosive.
- If there's a leak, you can smell it (much like why natural gas has additives).
- IT'S NOT EXPLOSIVE!
*Military ships convert it to a metal hydride form which, while bulkier, has the twin benefits of not exploding and serving as secondary armor.
** I am aware that ammonia is bulkier than liquid hydrogen and therefore NH3 contains less H per unit of volume, but since we're dealing with handwavey units of "H per dton" and it's universally applied across the board, I can claim that the required tonnage is for NH3 instead of LH. As long as everything is consistent, I don't think there's a problem here.
In conclusion
- If the ship has a life-support system, it can convert deuterium to ammonia and back.
- If it has a fuel processor, it can refine hydrogen to deuterium.
- Your pee helps make the ship go.



*blink*
ReplyDeleteMongo gaming brain just locked solid. Switching to alt personality matrix:
Damn, girl. She shoots loud guns, supports blog buddies in need, picks nommy profile pictures, and can sling gaming psuedo-scientific math.
Now I feel unworthy.
Ammonia is nasty stuff. Very corrosive. Why not just leave it as water? Especially since you're just using it as a Dt storage media.
ReplyDeleteI actually considered this, and really what it came down to was "Water in the tanks isn't nearly as useful for game purposes." There's no characteristic smell that your tanks have been punctured; there's no cascade of awful as they pour into the passenger compartment. Yes, water makes far more logical sense, but my writer and GM gut told me ammonia was more interesting.
ReplyDeleteFeel free to use water in your tanks, though.
Aw, shucks. :D
ReplyDeleteKeeping it as water means that you don't have as much processing time that is built into field refueling.
ReplyDeleteHonestly, the true time sink is sifting the hydrogen for deuterium. My earlier email to you guys was incorrect, because if you can fuse protons to create deuterium, you don't need to worry about D-D or D-T: just do straight P-P fusion until you get to Helium-3 and the process repeats. But this is the same process that stars use! If engines are going to be that efficient, it makes my under-educated brain wonder why the game mentions refined fuel in the first place -- after all, you can just toss anything into the Mister Fusion and you've got power.
ReplyDeleteI honestly think that the canonical time of 1-6 hours for frontier refueling is actually far too fast! Had I the skill I would devise some kind of formula for "How long does it it take to sort out X tons of Deuterium when a full tank is only 0.02% of all material sorted."
Hydrogen isn't that nasty (well, on earth it isn't). What it does is that it evaporates from pretty much any container, which makes it quite unsuitable on a spacecraft. Ammonia on the other hand IS nasty. VERY nasty. Actually it's one of the nastiest compound you come into regular contact with as a fireman (as it's used in all sorts of chemical industry). I'd suggest one of the hydrocarbons. Methane, propane etc.
ReplyDeleteReally? I did not know this.
ReplyDeleteStill, both methane and propane have the "goes boom" problem.
Assuming that the material to be refined consists of only hydrogen and naturally occuring deuterium, 1 ton of deuterium requires sorting through 5,000 tons of unrefined hydrogen.
ReplyDeleteIf the material to be refined contains hydrogen, first determine what percentage of the material is hydrogen - atomic mass if you have a compound in mind - for example, assuming ammonia with naturally occuring deuterium, such as might be found by a ship low on fuel in an otherwise uninhabited system, this is 3.03/17.03 or about 17.8% hydrogen. One ton of hydrogen extracted from otherwise pure ammonia requires about 5.62 tons of ammonia. Since you need five thousand tons of hydrogen to get one ton of deuterium, you would have to process 28,100 tons of ammonia to yield one ton of deuterium, assuming 100% efficiency.
Using triply-deuterated ammonia, this is simplified a great deal; the atomic mass of TDA is 20, 6 of which is deuterium, or 6/20, or 30%, so every 3.33 tons of TDA yields 1 ton of deuterium, also assuming 100% efficiency.
Using this approach, naturally occuring methane requires 19,865 tons of methane per ton of deuterium.
And ultimately the question comes down to how long do you WANT it to take? If it takes one hour to process 1000 tons of raw material at 100% efficiency, then using raw ammonia takes twenty eight hours, six minutes per ton of deuterium; TDA takes three hours, twenty minutes per ton of deuterium; and raw methane takes nineteen hours, fifty four minutes per ton of deuterium.
NH3 as bunny states is pretty evil. Its also known Anhydrous Ammonia, which is used as a fertilizer. How do you fertilize crops with a gas you ask? The Anhydrous part gives it away, it is desperately searching for hydro (water). Water in the soil moisture is ideal, but your eyes, lungs or any other mucus membranes are just as good. I would take explody gases transport over this one. I dont miss working with NH3 that much.
ReplyDeleteIt's not inconceivable that man-made fusion will run better than a star. It's engineered as opposed it "just happening". Compare the naturally occurring atomic reactions (http://en.wikipedia.org/wiki/Natural_nuclear_fission_reactor) to a power plant or Hiroshima.
ReplyDeleteThe reason there was refined fuel in OG Traveller was game balance. To give the party a reason to hit the star town. My OG game the unrefined fuel was something that gummed up the fuel lines and injectors. It wasn't that you couldn't burn the stuff, it was hard on the equipment. Clogged filters and cryogenic fluid fun time!
And why limit to just natural Dt? The power plant itself is a huge source of all sorts of radiation with spare neutrons galore. Breeder fusion reactor as it were.
There is also no reason to not burn the oxygen from water in the reactor either, it's still giving net positive energy (that's true until you start trying to burn iron in fact).
If you're going to use ammonia for your fuel you're going to need damn good double talk to justify it because it will have to be the absolute BEST choice for fuel bar none because otherwise there's no good reason to trap yourself in a sealed box with such corrosive nastiness.
Too much trouble, I think I'll be going fission and enjoying life at a slower pace...
ReplyDeleteDid you mean to put that negative exponent on the Joules? If so, that is a small amount of power as the negative exponent moves the decimal to the LEFT. If you meant 10 to the 12th instead of 10 to the negative 12th, then you just have a typo. (I'm too lazy to find a converter from electron-volts to Joules that works on my iPad right now or I'd be able to answer my own question.)
ReplyDeleteYes, it's a small amount of power, but that's *per reaction*. How many reactions are occurring every second? Lots, I imagine.
ReplyDelete4 grams (or rather, 2 moles) of deuterium yields 4.681664x10^12 joules, or about four and a half terajoules, if my math is turning out properly.
ReplyDeleteDepending on how efficient the reaction is, and how much power they actually need... but then, fusion reactions also require a significant input of energy to force the deuterium to interact with each other. Depending on how much energy this requires given the technology, much of the fuel consumption is to produce the energy to keep the reaction going.
The thing is that hydrocarbons will only burn if they have a proper fuel/air mixture (and they will only explode in a very narrow band of that fuel/air mixture). A propane tank will not go "boom" unless it's very close to empty. Handling it in space would be quite easy. Place the main propanetanks so that they can be:
ReplyDelete1. Vented into space.
2. Either be ejected into space (in an emergency) and/or with a construction that incorperates them into the hull so that any explosion will direct the force out into space.
Ammonia on the other hand. It's not only corrosive and poisonous (so poisonous that you can die within a few seconds of concentrated exposure) but moisture free it's a lighter than air gas, but combines easily with moisture to become a heavier than air gas. So by it's very nature any ammonia leak is unpredictable but tend to be as nasty as possible to any human around.
*blink*
ReplyDeleteMath is hard.
I honestly can't tell if you're being sarcastic or not, but just in case... I have made it plain from the beginning that my artsy-fartsy brain is good with abstract notions but tends to vapor lock at anything harder than arithmetic. It's not a gender thing, it's an "Erin is functionally innumerate which is why she's a writer and not an engineer."
ReplyDeleteWell, I was more going for "funny" than "sarcastic". :o
ReplyDeleteThe reasons for my suckage at math in school are all directly attributable to me myself and I, but I sympathize. (I kinda wanted to be an aeronautical engineer until I learned it involved maths and not just drawing pretty airplanes. :o )
Ah, okay. Thanks for that. :)
ReplyDeleteHere is what is sadly funny: I can understand theory like nobody's business, but the moment you pull out the equations my eyes glaze over, my mind goes blank and drool comes out the corner of my mouth. I can grasp the abstractions of a lot of high-end physics, but don't ask me to prove it mathematically. The sectors of the disk just *aren't there*.
For me, I blame the fact that I already knew how to read when I got to school, so I didn't actually have to *learn* anything in school until they dragged out long division and two'n'three digit multiplication in the fourth grade or whenever, and I was all "Huh?" But by then, I didn't really know how to study, so I just kinda derped my way through whatever math they made me do and squinched my eyes shut until it stopped. :o
ReplyDeleteIt's my understanding, based upon imperfect research, that if you have the ability to breed Deuterium from hydrogen then you don't actually need the deuterium, since you are engaging in proton-proton fusion. If you can find me some handwavey reason for how/why a reactor can make deut without being P-P, I will kiss you.
ReplyDeleteMy take on Traveller and fuel is:
ReplyDelete1. Power plant fuel is deuterium, but given the quantities used by the average ship this is a minor consideration. Refueling is part of the annual maintenance process or a mcguffin plot device in emergencies.
2. Maneuver drive fuel doesn't exists for most ships; thrusters are reactionless.
3. Jump 'fuel' isn't fuel per se, it's hydrogen used to generate a 'bubble' around the ship to protect it from Jumpspace effects. It's vented during the Jump initiation and partially ionised. Jump fuel is produced by cracking water, methane, ammonia or other hydrides using the fuel processor.